![]()
Chromium is a chemical element which has the symbol Cr and atomic number 24. The name of the element is derived from the Greek word "chroma" (χρῶμα), meaning colour, because many of its compounds are intensely coloured. Chromium oxide was used by the Chinese in the Qin dynasty over 2,000 years ago to coat metal weapons that have been found with the Terra cotta Army. Chromium was discovered as an element after it came to the attention of the western world in the red crystalline mineral crocoite. Crocoite was discovered in 1761 and initially used as a pigment. Louis Nicolas Vauquelin first isolated chromium metal from crocoite in 1797. Since Vauquelin's first production of metallic chromium, small amounts of native chromium metal have been discovered in rare minerals, but these are not used commercially. Instead, nearly all chromium is commercially extracted from the single commercially viable ore chromite, which is iron chromium oxide. Chromite is also now the chief source of chromium for chromium pigments. Chromium metal and ferrochromium alloy are commercially produced from chromite by silicothermic or aluminothermic reactions, or by roasting and leaching processes. Chromium metal has proven of high value due to its high corrosion resistance and hardness. A major development was the discovery that steel could be made highly resistant to corrosion and discolouration by adding metallic chromium to form stainless steel. This application, along with chrome plating, currently comprise 85% of the commercial use for the element, with applications for chromium compounds forming the remainder.
![]()
Within the metaphysical realm of minerals, chromium encourages mental regeneration and healing. It takes away feeling of being under pressure. The richness of the world of ideas and creative imagination can be discovered and versatile ideas and enthusiasm are the result. Chromium brings colour into the believer's life. Spiritual Chromium encourages the desire for self-determination and individuality. It stimulates the believer to discover and develop their own capabilities and to realize those's important dreams in life. Chromium promotes all processes of mental healing.
Please note that MIROFOSS does not suggest in any way that minerals should be used in place of proper medical and psychological care. This information is provided here as a reference only.
![]() Chromium is mainly used in the manufacture of stainless steel as well as chromium plating. Chromium can also be used as a colour pigment.
Chromium is mainly used in the manufacture of stainless steel as well as chromium plating. Chromium can also be used as a colour pigment.
![]()
Chromium is commonly found in the ore chromite as well as other chromium bearing minerals.
![]()
Chromium is the first element in Group 6 on the periodic table of elements. It is a steely-gray to white, lustrous, hard and brittle metal which takes a high polish, resists tarnishing, and has a high melting point. It is also odourless and tasteless. Trivalent chromium (Cr(III)) ion is possibly required in trace amounts for sugar and lipid metabolism, although the issue remains in debate. In larger amounts and in different forms, chromium can be toxic and carcinogenic. The most prominent example of toxic chromium is hexavalent chromium (Cr(VI)). Abandoned chromium production sites often require environmental cleanup.
![]()
| Colour | Silver, White | |
| Melting Point | 1907°C | |
| Boiling Point | 2671°C | |
| Specific Gravity | 7.19 | |
| Diaphaneity | Opaque | |
| Tenacity | Malleable | |
| Mohs Hardness | 8.5 | |
| Luminescence | non-fluroencent | |
| Lustre | Metallic | |
| Atomic Weight | 51.9961 | |
| Crystal Structure | Isometric | |
| Radioactivity | Non-radioactive | |
| Magnetism | antiferromagnetic at room temperature, paramagnetic Above 38 C | |
| Electrical Resistively | 125 nΩ·m At 20°C | |
| Thermal Conductivity | 93.9 W·m−1·K−1 |
![]()
The following health hazards should be noted when handling chromium
 |
BIOHAZARD Chromium contains the carcinogenic and mutagenic chromate ions. |
 |
ENVIORNMENTAL HAZARD |
![]()
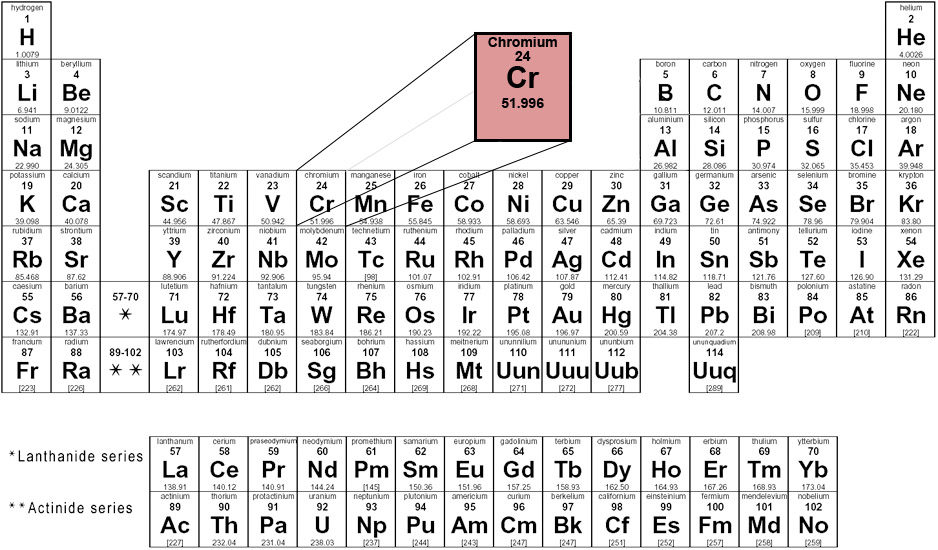
The following image shows where chromium appears on the periodic table of elements
Group 6 - Period 4 - Block d
![]()
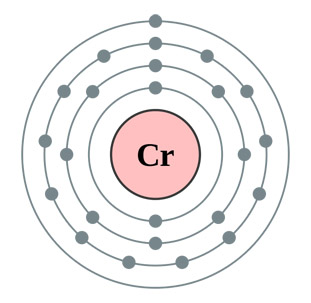 |
The diagram on the left shows the electron configuration of the element chromium. | |
| Oxidation States | 6, 5, 4, 3, 2, -1, -2 | |
| Electronegativity | 1.66 (pauling scale) | |
| Ioniazation energies | 1st: 652.9 kJ·mol−1 | |
| 2nd: 1590.6 kJ·mol−1 | ||
| 3rd: 2987 kJ·mol−1 | ||
| 4th: 4743 kJ·mol−1 | ||
| 5th: 6702 kJ·mol−1 | ||
| 6th: 8744.9 kJ·mol−1 | ||
| 7th: 15455 kJ·mol−1 | ||
| 8th: 17820 kJ·mol−1 | ||
| 9th: 20190 kJ·mol−1 | ||
| 10th: 23580 kJ·mol−1 | ||
| Atomic radius | 128 pm | |
| Covalent radius | 139±5 pm | |
![]()
Chromium cannot be referenced in current and historical texts under any other names
The element chromium can be translated into the following select languages:
| Arabic | كروم | Bulgarian | Хром | Chinese (Sim) | 自然铬 |
| Croatian | Krom | Czech | Chróm | Danish | Krom |
| Dutch | Chroom | Esperanto | Kromo | Estonian | Kroom |
| Finnish | Kromi | French | Chrome | German | Chrom |
| Greek | Χρώμιο | Hindi | क्रोमियम | Hungarian | Króm |
| Italian | Cromo | Japanese | クロム 自然クロム | Korean | 크로뮴 |
| Latin | Chromium | Lithuanian | Chromas | Norwegian | Krom |
| Persian | کروم | Polish | Chrom | Portuguese | Crômio |
| Romanian | Crom | Russian | Хром | Slovak | Chróm |
| Spanish | Cromo | Swedish | Krom | Tagalog | Cromo |
| Thai | โครเมียม | Ukrainian | Хром | Vietnamese | Crom |
![]()
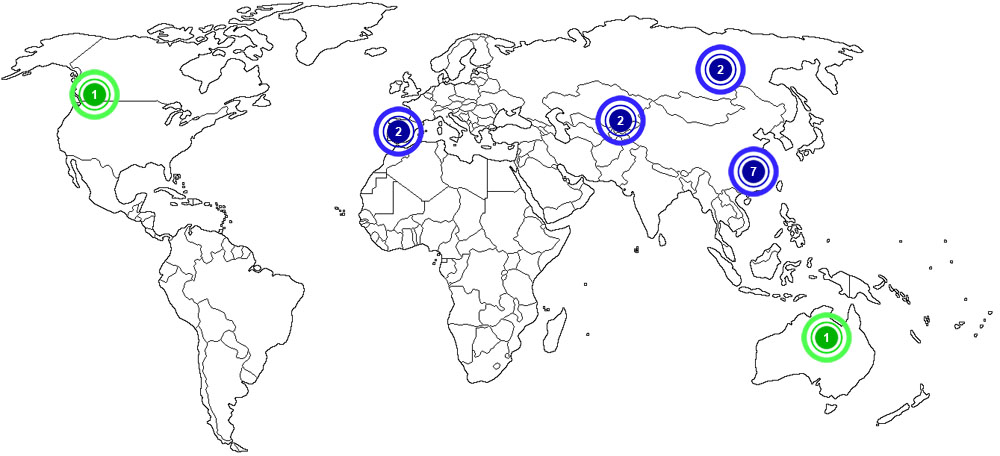
The map below shows the top chromium producing countries in the world as of 2014:
![]()
 |
The MIROFOSS database offers free printable geological identification tags for personal and non-profit use. These tags can be used to properly identify mineral samples in your collection. -Click here- to download a full size jpeg image for a chromium identification tag; which can be printed on paper or used with a plastic laser printer. |
 |
What's this? What can I do with it? |
![]()
| History | Dennis, J. K.; Such, T. E. (1993). "History of Chromium Plating". Nickel and Chromium Plating. Woodhead Publishing. pp. 9–12. |
| History | Guertin, Jacques; Jacobs, James Alan and Avakian, Cynthia P. (2005). Chromium (VI) Handbook. CRC Press. pp. 7–11. |
| History | Emsley, John (2001). "Chromium". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 495–498. |
| History | National Research Council (U.S.). Committee on Biologic Effects of Atmospheric Pollutants (1974). Chromium. National Academy of Sciences. p. 155. ISBN 978-0-309-02217-0. |
| History | Yue Suqin, Wang Wenying, and Sun Suqiong (1981): A new mineral--Native chromium. Chinese Science Bulletin [Kexue Tongbao] 26(15), 959-60. - American Mineralogist (1982), 67, 854-55 (abstract). |
| Physical Identification | Mindat.org. Retrieved on 2013-04-25 |
| June 24, 2014 | The last time this page was updated |
| ©2018 MIROFOSS™ Foundation | |
 |
|