![]()
Baryte, or barite, is a mineral consisting of barium sulfate. The baryte group of minerals consists of baryte, celestine, anglesite and anhydrite. Baryte itself is generally white or colourless, and is the main source of barium. The radiating form of baryte, sometimes referred to as Bologna Stone, attained some notoriety among alchemists for the phosphorescent specimens found in the 17th century near Bologna by Vincenzo Casciarolo. The name baryte is derived from the Greek word βαρύς (heavy). The American spelling barite is used by the United States Geological Survey and more often used in modern Scientific journals including those published by the Netherlands-based Elsevier journals. The International Mineralogical Association adopted "barite" as the official spelling when it formed in 1959, but recommended adopting the older "baryte" spelling in 1978.
![]()
Within the metaphysical realm of minerals, Baryte stimulates ones incentive to go for their dreams and goals without restraint, enhances relationship connections in friendship and love. Baryte can helps one to release trapped emotions and feelings, enhances self-assurance, helps to provide a strong, yet delicate connection to the spiritual world. Baryte helps link one to ones highest purpose and higher self. It acts as a magnet to high frequency energy, and activates and clears blockages in the upper chakras. Baryte assists one in recognizing where one must let go and allow change in order for evolution to occur. It facilitates memory and information retention and generally supports brain health.
Please note that MIROFOSS does not suggest in any way that minerals should be used in place of proper medical and psychological care. This information is provided here as a reference only.
![]() Historically, baryte was used for the production of barium hydroxide for sugar refining, and as a white pigment for textiles, paper, and paint. Today baryte is mainly used as a weighting agent for drilling fluids in oil and gas exploration to suppress high formation pressures and prevent blowouts. Other uses for baryte are in added-value applications which include filler in paint and plastics, sound reduction in engine compartments, coat of automobile finishes for smoothness and corrosion resistance, friction products for automobiles and trucks, radiation-shielding cement, glass ceramics and medical applications.
Historically, baryte was used for the production of barium hydroxide for sugar refining, and as a white pigment for textiles, paper, and paint. Today baryte is mainly used as a weighting agent for drilling fluids in oil and gas exploration to suppress high formation pressures and prevent blowouts. Other uses for baryte are in added-value applications which include filler in paint and plastics, sound reduction in engine compartments, coat of automobile finishes for smoothness and corrosion resistance, friction products for automobiles and trucks, radiation-shielding cement, glass ceramics and medical applications.
![]()
Baryte is commonly found as a gangue mineral in metallic ore deposits of epithermal or mesothermal origin; but it may also be found as lenses or replacement deposits in sedimentary rocks, both of hypogene and supergene origin.
![]()
Baryte occurs in a large number of depositional environments, and is deposited through a large number of processes including biogenic, hydrothermal, and evaporation. Baryte commonly occurs in lead-zinc veins in limestone, in hot spring deposits, and with hematite ore. It is often associated with the minerals anglesite and celestine. It has also been identified in meteorites. Baryte can be found in China, India, Morocco, Peru, Chile, Liberia, Turkey, Thailand, Ireland, Canada, The United States, Iran, Brazil, The United Kingdom, Greece, and Barberton Mountain Land, South Africa. Although baryte contains a heavy metal (barium), it is not considered to be a toxic chemical by most governments because of its extreme insolubility.
Other than in the basic mineral form, baryte can be found in twelve distinct varieties:
![]()
| Cleavage | Perfect to Imperfect | |
| Colour(s) | White, Yellowish white, Grayish white, Brownish white, Brown, Brownish red | |
| Specific Gravity | 4.48 | |
| Diaphaneity | Translucent to Transparent to Opaque | |
| Fracture | Uneven | |
| Mohs Hardness | 3.0 to 3.5 | |
| Luminescence | Phosphorescent | |
| Luster | Vitreous | |
| Streak | White | |
| Habit(s) | Massive to Prismatic to Tabular | |
| Radioactivity | Non-radioactive | |
| Magnetism | Diamagnetic |
![]()
No known health risks have been associated with baryte. However ingestion of baryte, as with other naturally occurring minerals, is not recommended.
![]()
The following image shows the Elemental breakdown of the mineral baryte along with the mineral crystal structure.
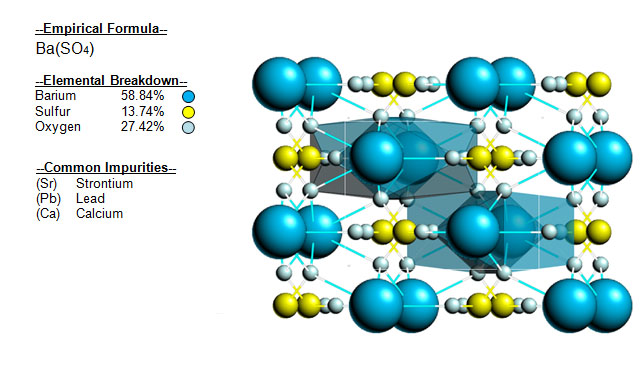
![]()
| Crystal System | Orthorhombic |  |
| Class | Dipyramidal | |
| Axial Ratios | a : b : c = 1.6289 : 1 : 1.3122 | |
| Optical Data Type | Biaxial (+) | |
| Pleochroism (x) | Colourless | |
| Pleochroism (y) | Colourless | |
| RL Values | nα = 1.634 - 1.637 nβ = 1.636 - 1.638 nγ = 1.646 - 1.648 | |
| 2V | Measured: 36° to 40°, Calculated: 36° to 42° |  |
| Max Birefringence | δ = 0.012 (See colour chart at right) | |
| Surface Relief | Moderate | |
| Dispersion | Weak r > v | |
| Notes: |
![]()
Baryte can be referenced in certain current and historical texts under the following twenty four names:
The mineral baryte can be translated into the following select languages:
| Arabic | باريت | Bulgarian | барит | Chinese (Sim) | 重晶石 |
| Croatian | Czech | Baryt | Danish | baryt | |
| Dutch | bariet | Esperanto | Estonian | bariit | |
| Finnish | baryyttia | French | Barite | German | Baryt |
| Greek | βαρυτίνη | Hebrew | בריט | Hungarian | Barit |
| Italian | Barite | Japanese | 重晶石 | Korean | 중정석 |
| Latin | Gypsum irregulare | Lithuanian | Baritas | Norwegian | barytt |
| Persian | سولفات باریم طبیعی | Polish | barytu | Portuguese | Barita |
| Romanian | Baritină | Russian | Барит | Slovak | Barit |
| Spanish | Barita | Swedish | Baryt | Tagalog | |
| Turkish | Barit | Ukrainian | Барит | Vietnamese |
![]()
Baryte is considered to be very abundant in certain areas of the world. The map below shows major documented concentrations of baryte:
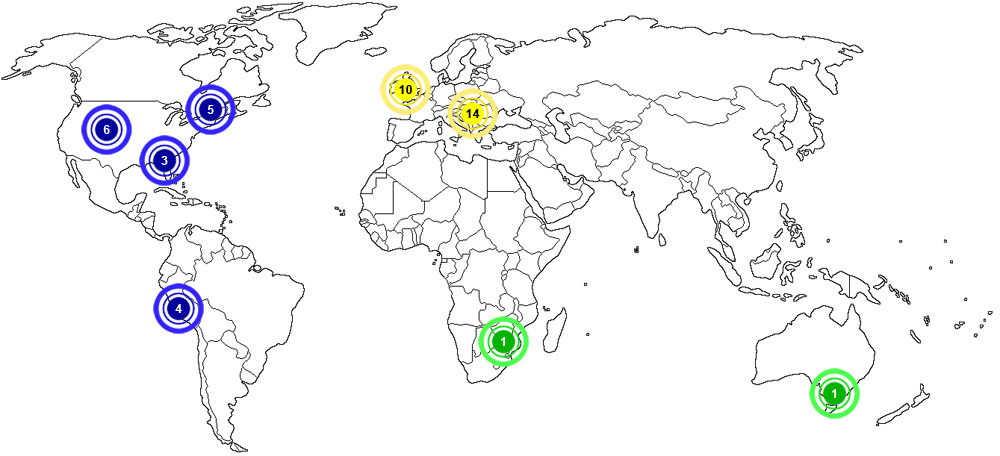

![]()
 |
The MIROFOSS database offers free printable geological identification tags for personal and non-profit use. These tags can be used to properly identify mineral samples in your collection. -Click here- to download a full size jpeg image for a baryte identification tag; which can be printed on paper or used with a plastic laser printer. |
 |
What's this? What can I do with it? |
![]()
| Chemical Composition | Majzlan, J., Navrotsky, A., and Neil, J.M. (2002) Energetics of anhydrite, barite, celestine, and anglesite: a high-temperature and differential scanning calorimetry study. Geochimica et Cosmochimica Acta: 66: 1839-1850. |
| Crystallography | American Mineralogist (1978): 63: 506-510. |
| History | Anthony, J.W., Bideaux, R.A., Bladh, K.W., and Nichols, M.C. (2003) Handbook of Mineralogy, Volume V. Borates, Carbonates, Sulfates. Mineral Data Publishing, Tucson, AZ, 813pp.: 45. |
| History | Gaines, Richard V., H. Catherine, W. Skinner, Eugene E. Foord, Brian Mason, Abraham Rosenzweig (1997), Dana's New Mineralogy : The System of Mineralogy of James Dwight Dana and Edward Salisbury Dana, 8th. edition: 572. |
| Geograpcial Data | Mindat.org. Retrieved on 2013-02-04 |
| Physical Identification | Webmineral.com. Retrieved on 2013-02-04. |
| June 07, 2012 | The last time this page was updated |
| ©2019 MIROFOSS™ Foundation | |
 |
|












