
Aluminium is the third most abundant element known on Earth after oxygen and silicon. Aluminium is the most abundant metal in the Earth's crust making up approximately 8%, by weight, of the Earth's solid surface. Aluminium metal is so chemically reactive that native specimens are rare and limited to extreme reducing environments. Instead, it is found combined in over 270 different minerals. The chief ore of aluminium is bauxite. Despite its prevalence in the environment, aluminium salts are not known to be used by any form of life. In keeping with its pervasiveness, aluminium is well tolerated by plants and animals. Ancient Greeks and Romans used aluminium salts as dyeing mordant and as astringents for dressing wounds; alum is still used as a styptic. In 1761, Guyton de Morveau suggested calling the base alum alumine. In 1808, Humphry Davy identified the existence of a metal base of alum, which he at first termed alumium and later aluminium. Aluminium was first produced in 1825 in an impure form by Danish physicist and chemist Hans Christian Ørsted. He reacted anhydrous aluminium chloride with potassium amalgam, yielding a lump of metal looking similar to tin. Friedrich Wöhler was aware of these experiments and cited them, but after redoing the experiments of Ørsted he concluded that this metal was pure potassium. He conducted a similar experiment in 1827 by mixing anhydrous aluminium chloride with potassium and yielded aluminium. Wöhler is generally credited with isolating aluminium but also Ørsted can be listed as its discoverer. Further, Pierre Berthier discovered aluminium in bauxite ore and successfully extracted it.
Within the metaphysical realm of minerals, aluminium is a projective energy that will shield your own energy. It's is in a sense, an invisibility cloak that deflects energy being sent your way. In jewelry, it will always protect the wearer. The planet associated with Aluminium is Mercury and it's Element is Air. Aluminium also enhances mental abilities such as intellect and telepathy as well as protects during astral travel.
Please note that MIROFOSS does not suggest in any way that minerals should be used in place of proper medical and psychological care. This information is provided here as a reference only.
Aluminium has a wide range of uses some of which include transportation parts for automobiles and aerospace, packaging, construction materials, household items, tools, and electrical transmission lines.
Tantalite is usually found in pegmatite and alluvial deposits.
Aluminium is a relatively soft, durable, lightweight, ductile and malleable metal with appearance ranging from silvery to dull grey, depending on the surface roughness. It is nonmagnetic and does not easily ignite. A fresh film of aluminium serves as a good reflector of light. The yield strength of pure aluminium is 7–11 MPa, while aluminium alloys have yield strengths ranging from 200 MPa to 600 MPa. Aluminium has about one-third the density and stiffness of steel. It is easily machined, cast, drawn and extruded. Corrosion resistance in aluminium can be excellent due to a thin surface layer of aluminium oxide that forms when the metal is exposed to air, effectively preventing further oxidation. The strongest aluminium alloys are less corrosion resistant due to galvanic reactions with alloyed copper. This corrosion resistance is also often greatly reduced by aqueous salts, particularly in the presence of dissimilar metals.
| Colour |
Silver, Grey |
| Melting Point |
660.32°C |
| Boiling Point |
2519°C |
| Diaphaneity |
Opaque |
| Tenacity |
Malleable |
| Mohs Hardness |
2.0 to 3.5 |
| Luminescence |
non-fluroencent |
| Lustre |
Metallic |
| Atomic Weight |
26.9815386 |
| Crystal Structure |
Isometric |
| Radioactivity |
Non-radioactive |
| Magnetism |
Non-magnetic |
| Electrical Resistivity |
28.2 nΩ·m at 20°C |
| Thermal Conductivity |
205 W·m−1·K−1 |
No known health risks have been associated with aluminium. However ingestion of aluminium, as with other naturally occurring elements, is not recommended.
The following image shows where aluminium appears on the periodic table of elements.
Group 13 - Period 3 - Block p
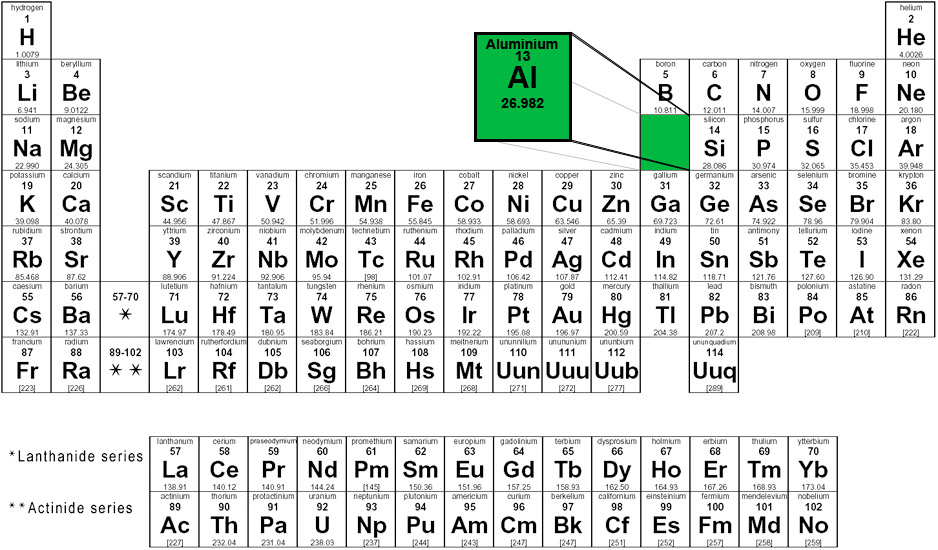
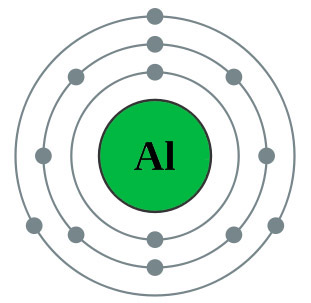 |
The diagram on the left shows the electron configuration of the element aluminium. |
| |
| Oxidation States |
3, 2, 1 |
| Electronegativity |
1.61 (pauling scale) |
| Ionization energies |
1st: 577.5 kJ·mol−1 |
| |
2nd: 1816.7 kJ·mol−1 |
| |
3rd: 2744.8 kJ·mol−1 |
| |
4th: 11577 kJ·mol−1 |
| |
5th: 14842 kJ·mol−1 |
| |
6th: 18379 kJ·mol−1 |
| |
7th: 23326 kJ·mol−1 |
| |
8th: 27465 kJ·mol−1 |
| |
9th: 31853 kJ·mol−1 |
| |
10th: 38473 kJ·mol−1 |
| Atomic radius |
143 pm |
| Covalent radius |
121±4 pm |
| The band of colour shown below is the spectral line of the element aluminium. |
 |
|
|
Aluminium cannot be referenced in certain current and historical texts under any other names:
The element aluminium can be translated into the following select languages:
| Arabic |
ألومنيوم |
Bulgarian |
Алуминий |
Chinese (Sim) |
自然铝 |
| Croatian |
Aluminij |
Czech |
Hliník |
Danish |
Aluminium |
| Dutch |
Aluminium |
Esperanto |
Aluminio |
Estonian |
Alumiinium |
| Finnish |
Alumiini |
French |
Aluminium |
German |
Aluminium |
| Greek |
Αργίλιο |
Hebrew |
אלומיניום |
Hungarian |
Alumínium |
| Italian |
Alluminio |
Japanese |
自然アルミニウム |
Korean |
알루미늄 |
| Latin |
Aluminium |
Lithuanian |
Aliuminis |
Norwegian |
Aluminium |
| Persian |
آلومینیم |
Polish |
Glin |
Portuguese |
Alumínio |
| Romanian |
Aluminiu |
Russian |
Алюминий |
Slovak |
Hliník |
| Spanish |
Aluminio |
Swedish |
Aluminium |
Tagalog |
Aluminio |
| Turkish |
Alüminyum |
Ukrainian |
Алюміній |
Vietnamese |
Nhôm |
Aluminum is produced and recycled in many places around the world. The map below shows major documented concentrations of aluminum:
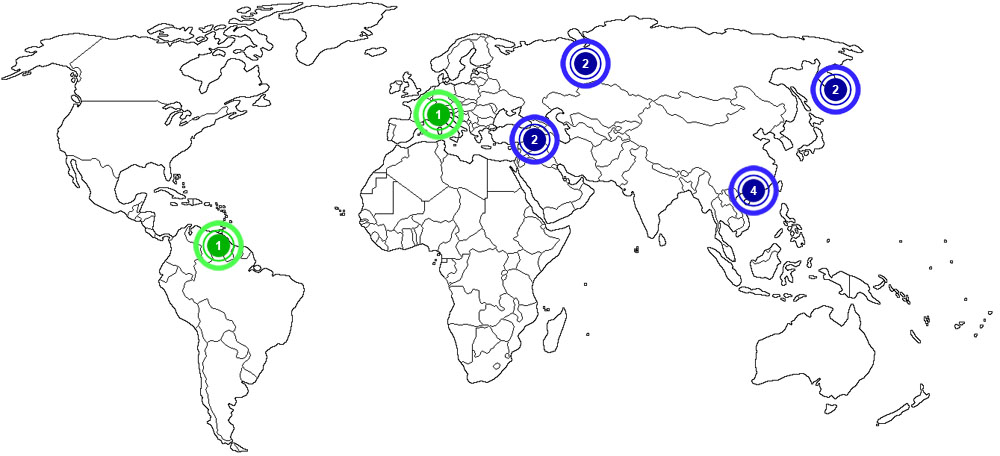
 |
The MIROFOSS database offers free printable geological identification tags for personal and non-profit use. These tags can be used to properly identify mineral samples in your collection. -Click here- to download a full size jpeg image for an aluminum identification tag; which can be printed on paper or used with a plastic laser printer. |
 |
What's this?
This is a QR code (short for Quick Response) which gives fast-track access to MIROFOSS articles. QR Codes are barcodes that can be read by smart phone cameras. This QR Code is unique to this MIROFOSS article.
What can I do with it?
You can copy and print the QR code to a plant label, poster, book, web site, magazines, or newspaper so smart phone users can scan the QR Code which automatically takes them to this specific article. |
| Chemical Composition |
Shakhashiri, B. Z. (17 March 2008). "Chemical of the Week: Aluminum" |
| Spectra Data |
Merrill, P. W.; Deutsch, A. J.; Keenan, P. C. (1962). "Absorption Spectra of M-Type Mira Variables |
| History |
Dohmeier, C.; Loos, D.; Schnöckel, H. (1996). "Aluminum(I) and Gallium(I) Compounds: Syntheses, Structures, and Reactions" |
| History |
"alumium", Oxford English Dictionary. Ed. J.A. Simpson and E.S.C. Weiner, second edition Oxford: Clarendon Press, 1989. OED Online Oxford University Press. |
| Physical Identification |
Mindat.org. Retrieved on 2012-08-29 |
| August 29, 2012 |
The last time this page was updated |
| ©2017 MIROFOSS™ Foundation |
|














