

| Mineral Name | Anhydrite |
| First Discovered | 1804 |
| Nickel-Strunz Classification | 07.AD.30 |
| Dana Classification | 28.03.02.01 |
| ICSD | 16382 |
| Mineral Group | Sulfates |


| Cleavage | Perfect to Good |
| Colour(s) | Colourless, White, Bluish white, Violet white, Dark grey |
| Specific Gravity | 2.97 |
| Diaphaneity | Translucent to Transparent |
| Fracture | Brittle |
| Mohs Hardness | 3.5 |
| Luminescence | Non-fluorescent |
| Lustre | Vitreous |
| Streak | White |
| Habit(s) | Fibrous to Granular to Plumose |
| Radioactivity | Non-radioactive |
| Magnetism | Non-magnetic |

No known health risks have been associated with anhydrite. However ingestion of anhydrite, as with other naturally occurring minerals, is not recommended.

The following image shows the Elemental breakdown of the mineral anhydrite along with the mineral crystal structure.
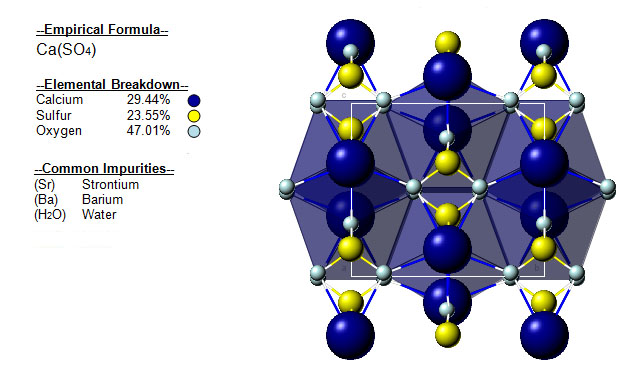

| Crystal System | Orthorhombic |  |
| Class | Dipyramidal | |
| Axial Ratios | a : b : c = 0.893 : 1 : 1 | |
| Optical Data Type | Biaxial (+) | |
| Pleochroism (x) | Colourless | |
| Pleochroism (y) | Colourless |  |
| RL Values | nα = 1.567 - 1.574 nβ = 1.574 - 1.579 nγ = 1.609 - 1.618 | |
| 2V | Measured: 36° to 45°, Calculated: 44° | |
| Max Birefringence | δ = 0.042 - 0.044 (See colour chart at right) | |
| Surface Relief | Low | |
| Dispersion | Strong r < v | |

Anhydrite can be referenced in certain current and historical texts under the following six names:
The mineral anyhdrite can be translated into the following select languages:
| Arabic | الأنهيدريت | Bulgarian | Ангидрит | Chinese (Sim) | 硬石膏 |
| Croatian | anhidrit | Czech | Anhydrit | Danish | anhydrit |
| Dutch | Anhydriet | Esperanto | Anhidrito | Estonian | Anhüdriitpinnad |
| Finnish | anhydriitti | French | Anhydrite | German | Anhydrit |
| Greek | ανυδρίτης | Hebrew | אנהידריט | Hungarian | Anhidrit |
| Italian | Anidrite | Japanese | 硬石膏 | Korean | 경석고 |
| Latin | Anhydrite | Lithuanian | Anhidritas | Norwegian | anhydritt |
| Persian | انیدریت | Polish | Anhydryt | Portuguese | Anidrita |
| Romanian | anhidrit | Russian | Ангидрит | Slovak | Anhydrit |
| Spanish | Anhidrita | Swedish | Anhydrit | Tagalog | anhidrit |
| Turkish | anhidrit | Ukrainian | Ангідрит | Vietnamese | thứ thạch cao cứng |

Anhydrite is considered to be very abundant around the world. The map below shows major documented concentrations of Anhydrite:
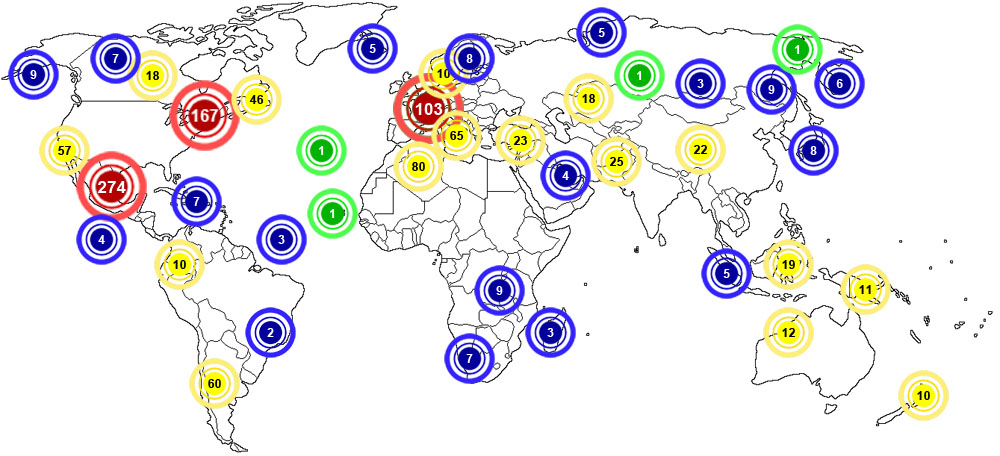

 |
The MIROFOSS database offers free printable geological identification tags for personal and non-profit use. These tags can be used to properly identify mineral samples in your collection. -Click here- to download a full size jpeg image for a anhydrite identification tag; which can be printed on paper or used with a plastic laser printer. |
 |
What's this? What can I do with it? |

| Chemical Composistion | Anthony, J.W., Bideaux, R.A., Bladh, K.W., and Nichols, M.C. (2003) Handbook of Mineralogy, Volume V. Borates, Carbonates, Sulfates. Mineral Data Publishing, Tucson, AZ, 813pp.: 25. |
| Crystallography | Hardie, L.A. (1967), The gypsum-anhydrite equilibrium at one atmosphere pressure: American Mineralogist: 52: 171-200. |
| History | Canadian Mineralogist (1975): 13: 289-292. |
| History | Palache, C., Berman, H., & Frondel, C. (1951), The System of Mineralogy of James Dwight Dana and Edward Salisbury Dana, Yale University 1837-1892, Volume II. John Wiley and Sons, Inc., New York, 7th edition, revised and enlarged: 407, 424-428. |
| Geographical Data | Mindat.org. Retrieved on 2013-02-07 |
| Physical Identification | Webmineral.com. Retrieved on 2013-02-07 |
| February 07, 2013 | The last time this page was updated |
| ©2017 MIROFOSS™ Foundation | |
 |